什麼是PGT-A檢測?
- PGT-A是一種對胚胎進行的染色體篩檢,用以辨識染色體數目是否異常(非整倍體)。
- 我們的次世代定序技術讓我們能夠分析全部24種染色體。在進行胚胎著床前偵測染色體異常,可在資訊充足的情況下做出決定,並提高懷孕成功率。
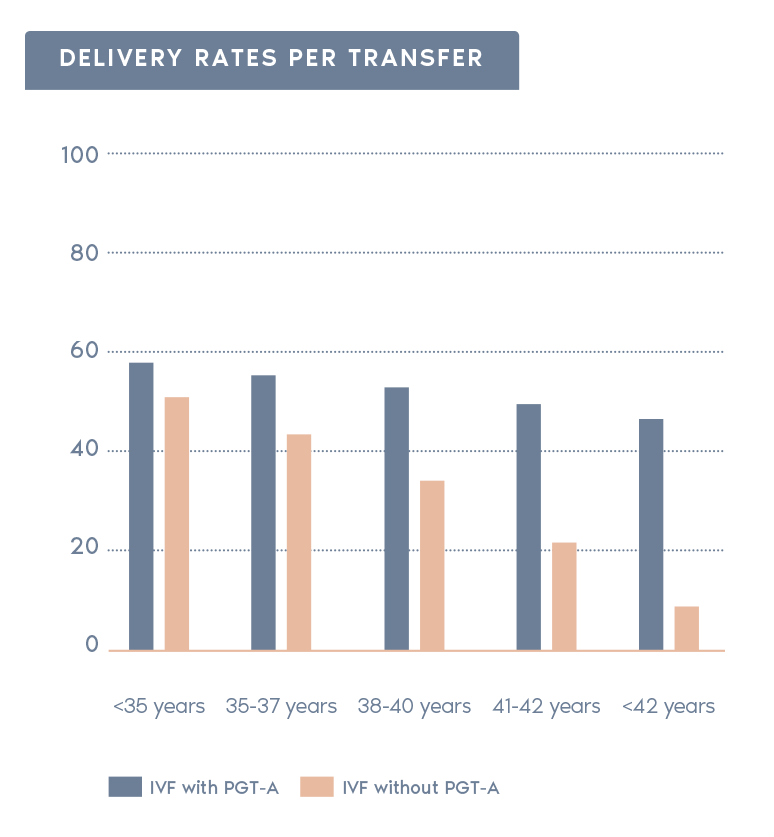
根據SART 2016公開資料庫的分析,PGT-A對臨床結果的影響
(包含進行與未進行PGT-A之比較)


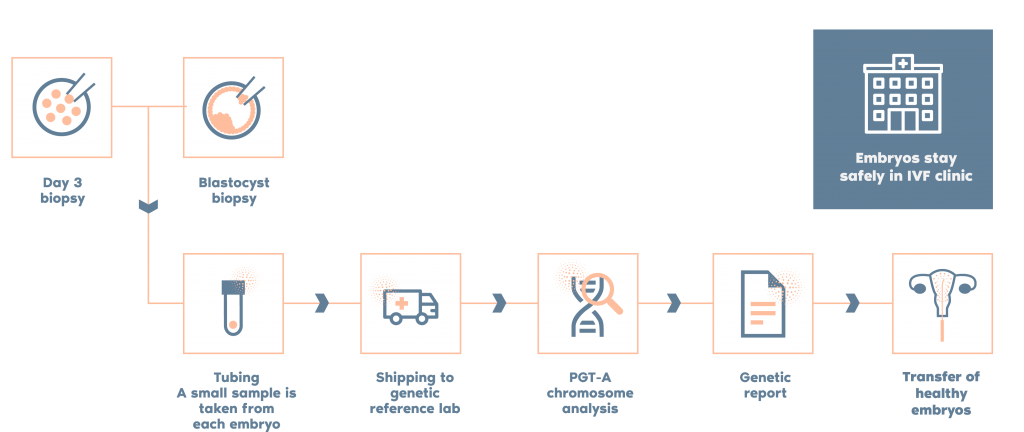
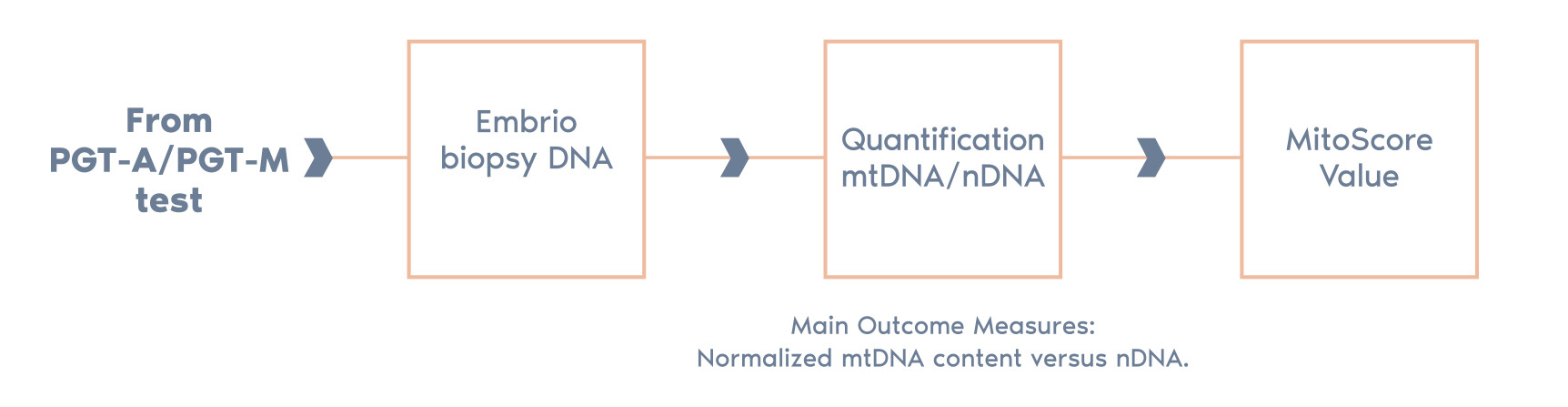
檢測流程




獨立研究比較 Igenomix 和其他實驗室的 PGT-A 檢測,關鍵差異如下:
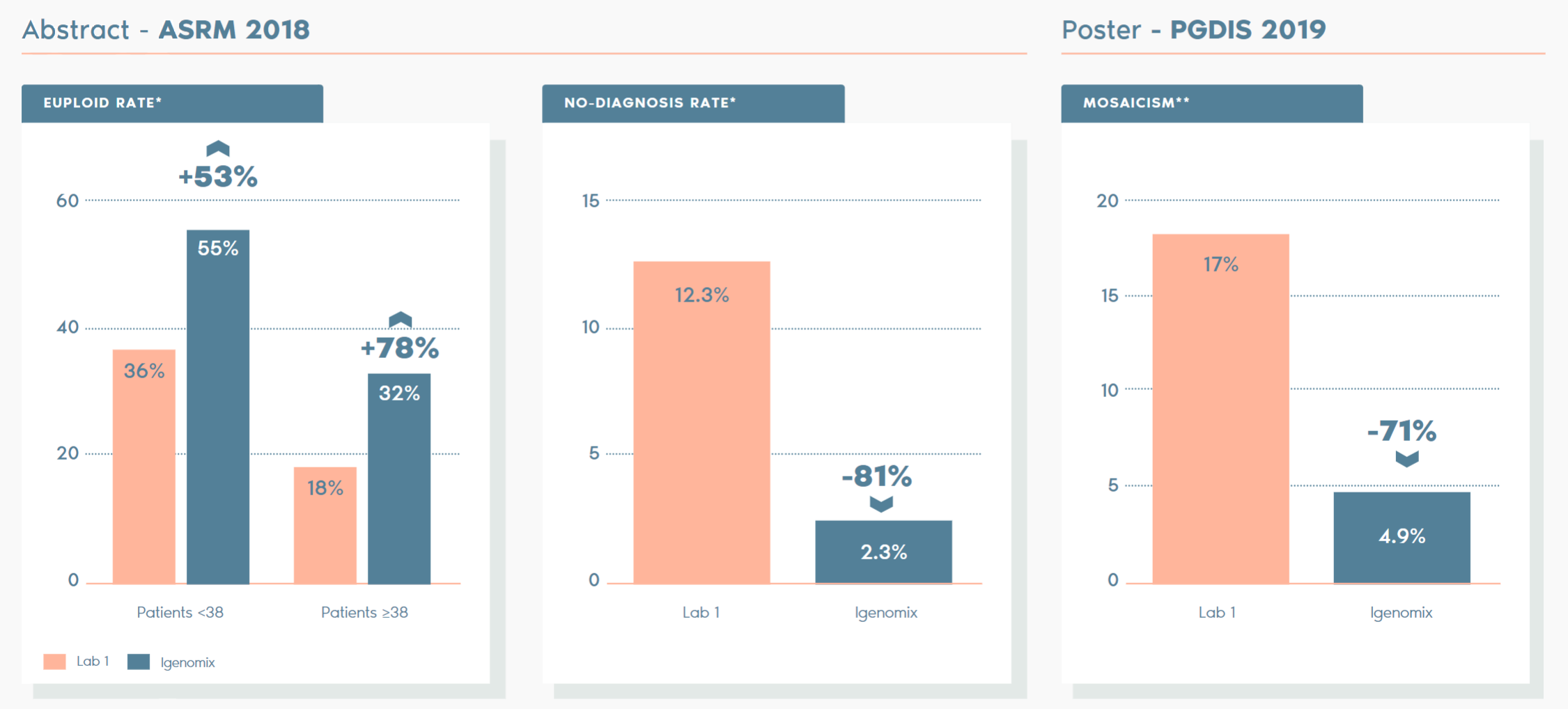
*ABSTRACT – ASRM 2018:對比在過渡期內來自參考實驗室的胚胎植入前基因檢測 (PGT-A) 診斷結果的研究;趨勢和對患者護理的影響。D. Ioannou, M. D. Baker, S. D. Jones, l. R. Grass, K. A. Miller. Embryology, IVF Florida Reproductive Associates, Margate, FL.
**POSTER – PGDIS 2019:利用不同閾值來確定倍性狀態的兩種 PGT-A 平台的臨床比較。作者:D. Monahan, G. Harton, D. Griffin, M. Angle, C. Smikle. Laurel Fertility Care, San Francisco, CA.
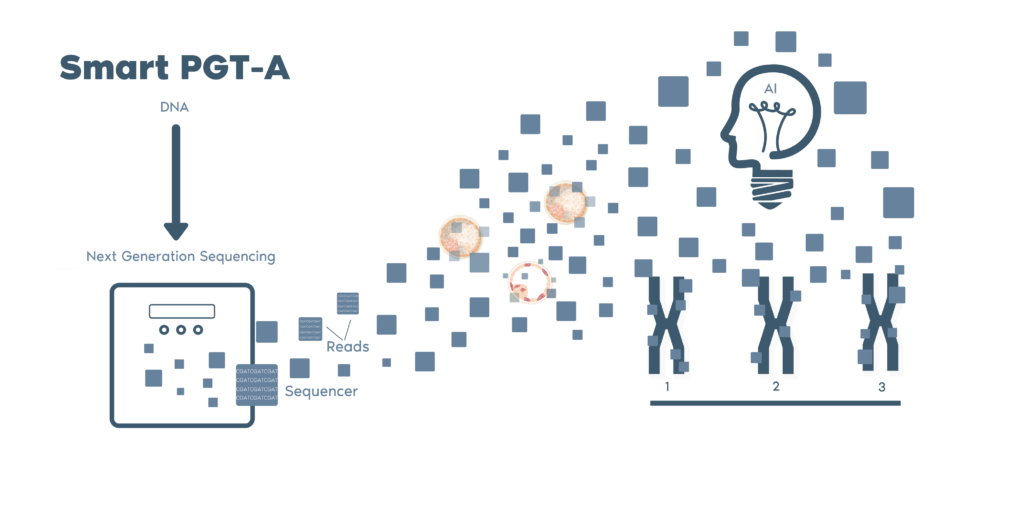
透過採用獨特 Smart PGT-A 技術,Igenomix 能夠不斷改進和提升我們分析胚胎樣本的能力。我們基於超過 100,000 個胚胎樣本開發了一種專有的生物資訊學辨識算法,這種算法能夠最大限度地減少人為錯誤和偏差/主觀性。
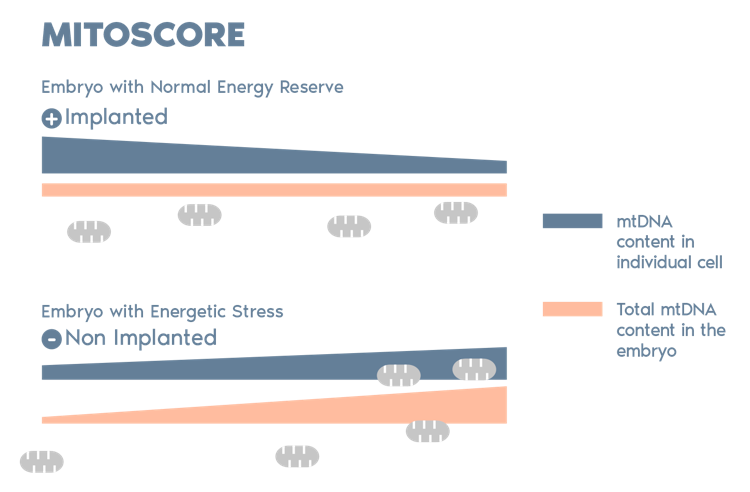
MitoScore是由Igenomix研發,一種基於粒線體 DNA (mtDNA) 儲備的生物標誌(mitochondrial biomarker),以此為一個胚胎活力狀態的指標。透過這種技術,Igenomix 能夠選擇那些具有最高植入潛力的胚胎,從而提高 IVF 和 PGT-A 治療的成功率。*
*(Diez-Juan et al. 2015)
PGT-A 無法測試:
PGT-A 測試有一些限制,如下列情況:
1. 準確度~98%
2. PGT-A只能檢測胚胎切片,而不是整個胚胎
3. 無法偵測與遺傳物質增減無關的染色體結構異常
建議後續接受產前檢測以更加確認 PGT-A 結果。
運送有可能會發生無法控制的問題,例如天氣和航空問題,或者其他超出Igenomix控制範圍的情況,使得Igenomix無法即時提供測試結果,以進行胚胎植入。
Igenomix 實驗室收到的樣本有可能不符合分析條件,導致無法取得測試結果。
在非常罕見的情況下,因不當切片技術、檢體遺失或者不良DNA品質,可能導致無法進行基因檢測。
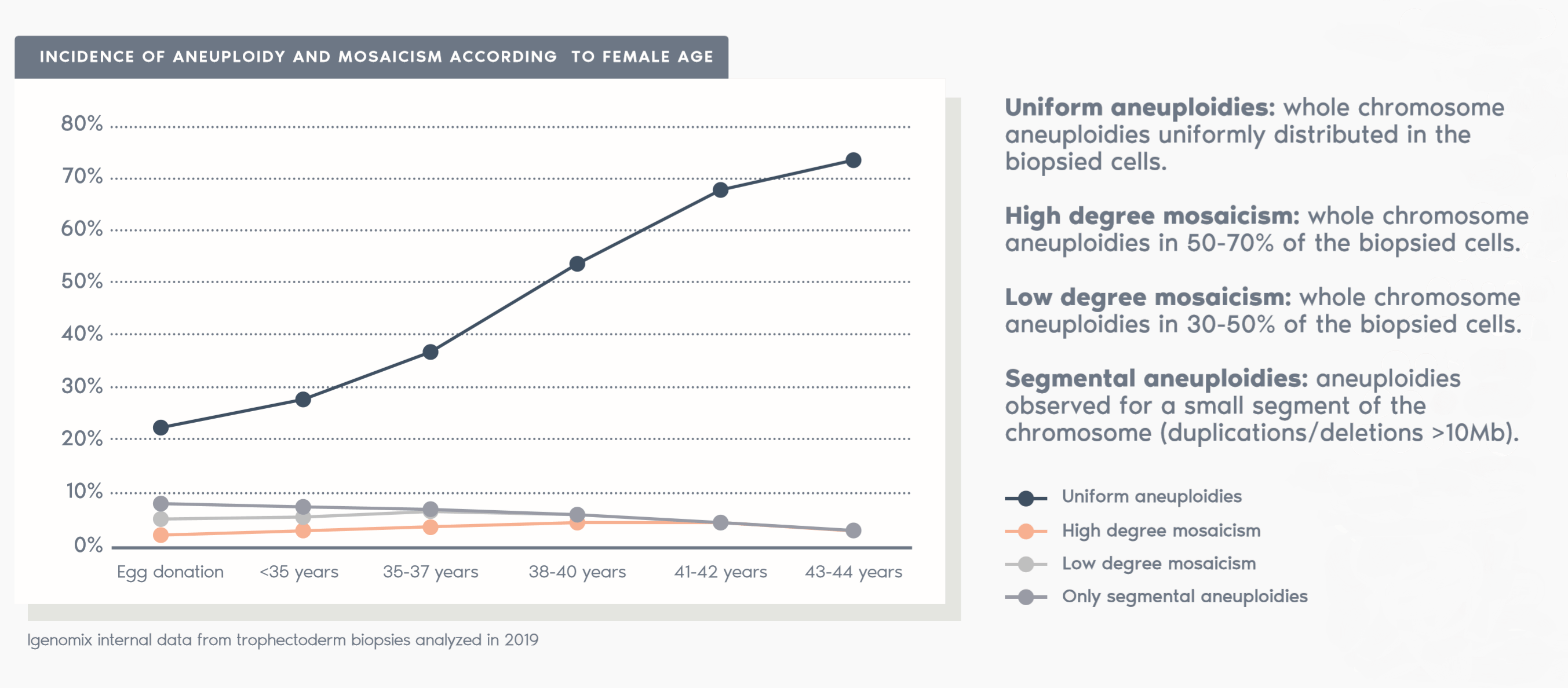
過去幾年,胚胎染色體鑲嵌現像一直是 ART 界爭論最多的議題之一。
鑲嵌現像在胚胎發育過程中隨機且自發性地發生。在細胞分裂過程中,染色體可能分佈不均勻,產生染色體數量異常的細胞。
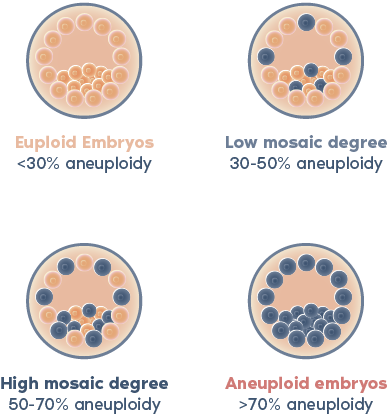
根據我們使用 NGS 對 PGT-A 進行的內部驗證,當活檢中檢測到的非整倍體水平大於 30% 且小於 70% 時,胚胎將被視為嵌合體。
如果活檢中的非整倍體水平在 50% 至 70% 之間,則胚胎將被視為具有「低水平嵌合體」。
據悉,低水平嵌合體的胚胎已被證明具有顯著的生殖潛力。
儘管一些研究表明它們可能具有較低的著床率和較高的流產風險(Viotti et al., 2021),但我們的非選擇研究顯示整倍體胚胎具有相似的臨床結果(Capalbo et al.,2021)。
最近發表的PGDIS 2021年期刊《胚胎異質性轉移的立場聲明》認為,低異質性胚胎的轉移對後代是一個相對安全的選擇。 (https://pgdis.org/docs/PositionStatement.pdf)
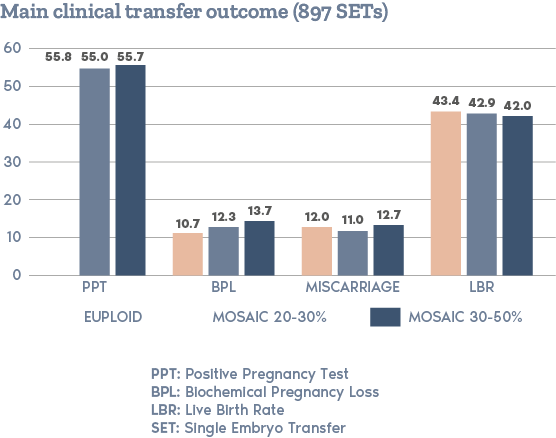
人們認為,嵌合體在整個懷孕期間或直到出生時持續存在的風險很低。
高水平嵌合體的胚胎可能具有一定的生殖潛力,但與整倍體和低水平嵌合體胚胎相較下,植入率較低、流產風險較高。
哪個胚胎適合植入,還是由患者和醫生共同決定。
Capalbo et al., Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial. Am J Hum Genet. 2021 Dec 2;108(12):2238-2247.
García-Pascual et al., Optimized NGS Approach for Detection of Aneuploidies and Mosaicism in PGT-A and Imbalances in PGT-SR. Genes 2020 Jul; 11(7):724.
Rodrigo et al., Characteristics of the IVF Cycle that Contribute to the Incidence of Mosaicism. Genes 2020 Oct; 11(10):1151.
Rubio et al: In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017 May;107(5):1122-1129.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet 2012 May 2; 5(1):24.
Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, Ata B, Cohen J, Munné S. Optimal euploid embryo transfer strategy, fresh versus frozen, after Preimplantation Genetic Testing for Aneuploidies with next generation sequencing: a randomized controlled trial. Fertil Steril. 2017 Mar;107(3):723-730.e3.
Coates A, Bankowski BJ, Kung A, Griffin DK, Munne S. Differences in pregnancy outcomes in donor egg frozen embryo transfer (FET) cycles following Preimplantation Genetic Testing for Aneuploidies (PGT-A): a single center retrospective study. J Assist Reprod Genet. 2017 Jan;34(1):71-78.
Viotti et al., Using outcome data from one thousand mosaic embryo transfers to formulate an embryo ranking system for clinical use. Fertil Steril. 2021 May;115(5):1212-1224.
Leigh et al., PGDIS position statement on the transfer of mosaic embryos 2021. Reprod Biomed Online. 2022 Mar 20;S1472-6483(22)00150-X.
Goldwaser, Tamar et al. Cell-free DNA for the detection of fetal aneuploidy. Fertility and Sterility, 2018; 109, 2, 195 – 200.
Kuznyetsov V, Madjunkova S, Antes R, Abramov R, Motamedi G, Ibarrientos Z, et al. Evaluation of a novel non-invasive preimplantation genetic screening approach. PLoS ONE; 2018; 13(5): e0197262.
Munné S, Status of preimplantation genetic testing and embryo selection. RBMO. 2018; 37(4):393-396.
Levy et al., Prenatal diagnosis by chromosomal microarray analysis. Fertility and Sterility, 2017; 109, 2, 201–212.
Ottolini C. et al., Scientific Reports. Tripolar mitosis and partitioning of the genome arrests human preimplantation development in vitro, 2017;7:9744.
Munné, Santiago et al. Mosaicism: “survival of the fittest” versus “no embryo left behind”. Fertility and Sterility, 2016; 105, 5, 1146 – 1149.
Bolton et al., Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nature Communications, 2016; 29;7:11165.
Cimadomo D. et al, The Impact of Biopsy on Human Embryo Developmental Potential during Preimplantation Genetic Diagnosis. Hindawi, 2016, 7193075.
Scott RT, Galliano D., The challenge of embryonic mosaicism in preimplantation genetic screening. Fertility and Sterility, 2016; 105, 5.
McCoy RC, Demko ZP, Ryan A, Banjevic M, Hill M, Sigurjonsson S, et al. Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development. PLoS Genet, 2015; 11 (10): e1005601.
Kung A. et al., Validation of next-generation sequencing for comprehensive chromosome screening of embryos. Reproductive BioMedicine, 2015; 1472-6483.
Greco E, Minasi MG, Fiorentino F. Healthy Babies after Intrauterine Transfer of Mosaic Aneuploid Blastocysts, N Engl J Med, 2015; Nov 19;373(21):2089-90.
Yang Z et al., Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study., 2012, 5:24.